40 open-label study o que é
Clinical trial - Wikipedia Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study ... Dietary Supplements: What You Need to Know - Consumer In addition to vitamins, dietary supplements can contain minerals, herbs or other botanicals, amino acids, enzymes, and many other ingredients. Dietary supplements come in a variety of forms, including tablets, capsules, gummies, and powders, as well as drinks and energy bars. Popular supplements include vitamins D and B12; minerals like ...
Semicircular canal size constrains vestibular function in miniaturized ... As endolymph flows through the tubular semicircular canals, its movement is governed by Poiseuille's law, which states that the flow (Q) of a fluid through a tube is directly proportional to the fourth power of the tube radius (r 4), and the pressure difference between the inflow (P i) and outflow (P o) of the tube and inversely proportional to the tube length (l) and the fluid viscosity (η ...

Open-label study o que é
Effect of Dexamethasone in Hospitalized Patients with COVID-19 ... Methods The Randomised Evaluation of COVID-19 therapy (RECOVERY) trial is a randomized, controlled, open-label, adaptive, platform trial comparing a range of possible treatments with usual care in patients hospitalized with COVID-19. We report the preliminary results for the comparison of dexamethasone 6 mg given once daily for up to ten days vs. usual care alone. H-E-B | Curbside Pickup & Grocery Delivery | HEB.com H-E-Buddy Sugar Free Kids Multi Vitamin Jelly Beans. Add to cart. Add to list. $5.03 each ($0.08 / ct) H-E-B Calcium with Vitamin D Gummy Kid Gummies Vitamins. Add to cart. Add to list. $8.98 each ($0.30 / ct) H-E-B Vitamins 1000 mg Vitamin C Orange Powder Drink Mix. Add to cart. Add to list. Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers and the ...
Open-label study o que é. Adjuvant atezolizumab after adjuvant chemotherapy in resected ... - PubMed Methods: IMpower010 was a randomised, multicentre, open-label, phase 3 study done at 227 sites in 22 countries and regions. Eligible patients were 18 years or older with completely resected stage IB (tumours ≥4 cm) to IIIA NSCLC per the Union Internationale Contre le Cancer and American Joint Committee on Cancer staging system (7th edition). Coloplast DialogueStudy - Full Text View - ClinicalTrials.gov None (Open Label) Primary Purpose: Treatment: Official Title: An Open Label, Non-comparative, Multi-national Post-Market Study to Document Real Life Experience on SenSura With Focus on Skin Condition and Quality of Life: Study Start Date : February 2008: Actual Primary Completion Date : April 2010: Actual Study Completion Date : December 2010 Google Tradutor O serviço gratuito do Google traduz instantaneamente palavras, frases e páginas da Web entre o inglês e mais de 100 outros idiomas. Tradutor. Fazer login. Tradutor. Sobre o Google Tradutor. Privacidade e Termos Ajuda Enviar feedback Sobre o Google. Tipos de tradução. translate Texto. Injectable poly-L-lactic acid for human immunodeficiency virus ... Materials and methods: This is an ongoing open-label, multicenter, 5-year study of 290 treated subjects. After correction with injectable PLLA, subjects are being followed annually. Primary end points include incidence and severity of treatment-emergent adverse events (TEAEs). Secondary end points include mean change from baseline of James ...
Talimogene Laherparepvec Improves Durable Response Rate in Patients ... This open-label study was conducted at 64 centers in the United States, the United Kingdom, Canada, and South Africa and overseen by an independent data monitoring committee. Patients were assigned at a two-to-one ratio using central random assignment to receive intralesional T-VEC or subcutaneous recombinant GM-CSF. Random assignment was ... PDF Effect of Convalescent Plasma on Mortality among Hospitalized ... - medRxiv 122 pragmatic intervention conducted as a multicenter, open-label protocol in hospitalized 123 adults with COVID-19. All hospitals or acute care facilities in the US and any physician ... protocol, FDA, and state regulations. 126 Mayo Clinic served as the academic research organization conducting the study. The 127 Mayo Clinic Institutional ... Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs ... - JAMA A major strength is the pragmatic design of the study and its novel primary end point, which was based on a symptom assessment questionnaire (time to reduction in symptom score by 50%). A major limitation was that there was no placebo control group; the current study was open label, and patients were not masked to which therapy they received. What is an open label extension study? • NCK Pharma An open – label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered. This contrasts with single blind and double blind experimental designs, where participants are not aware of what treatment they are receiving (researchers are also unaware in a double blind ...
PDF Consent to Participate in A Research Study and Research Subject Hipaa ... research team is going to talk to you about the research study, and they will give you this consent form to read. You may also decide to discuss it with your family, friends, or family doctor. You may find some of the medical language difficult to understand. If you agree to take part in this study, you will be asked to sign this consent form. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal ... Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer ... Open-label study | definition of open-label study by Medical ... open-label study: a study in which there is no blinding of treatments. MDMA - Multidisciplinary Association for Psychedelic Studies - MAPS Other names: MDMA, Ecstasy, E, X, XTC, Molly. Onset of action: 30-45 minutes (orally) Peak: 1.5 to 2 hours. See more See less. Read our Research. Research Resources. ... A Phase 1/2 open-label treatment development study of MDMA-Assisted Cognitive-Behavioral Conjoint Therapy (CBCT) in dyads in which one member has chronic posttraumatic stress ...
Hydroxychloroquine and azithromycin as a treatment of ... - ScienceDirect An information document that clearly indicates the risks and benefits associated with the participation to the study was given to each patient. Patients received information about their clinical status during care regardless of whether they participate in the study or not.
Medical Definition of Open-label - MedicineNet Reviewed on Privacy & Trust Info Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving.
Therapeutic use of intermittent fasting for people with type 2 diabetes ... This case series documents three patients referred to the Intensive Dietary Management clinic in Toronto, Canada, for insulin-dependent type 2 diabetes. It demonstrates the effectiveness of therapeutic fasting to reverse their insulin resistance, resulting in cessation of insulin therapy while maintaining control of their blood sugars. In addition, these patients were also able to lose ...
ICH Official web site : ICH The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is unique in bringing together the regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of pharmaceuticals and develop ICH guidelines. Since its inception in 1990, ICH has gradually evolved, to ...
Reduction of dietary sodium to less than 100 mmol in heart failure ... SODIUM-HF is an international, open-label, randomised, controlled trial that enrolled patients at 26 sites in six countries (Australia, Canada, Chile, Colombia, Mexico, and New Zealand). Eligible patients were aged 18 years or older, with chronic heart failure (New York Heart Association [NYHA] functional class 2-3), and receiving optimally ...
A Phase 1 Study to Evaluate the Safety and Immunogenicity of eOD-GT8 ... A Phase 1, Randomized, First-in-human, Open-label Study to Evaluate the Safety and Immunogenicity of eOD-GT8 60mer mRNA Vaccine (mRNA-1644) and Core-g28v2 60mer mRNA Vaccine (mRNA-1644v2-Core) in HIV-1 Uninfected Adults in Good General Health. The hypothesis is that sequential vaccination by a germline-targeting prime followed by directional ...
A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid ... An open-label study published in 2004 suggested, by comparison with a historical control group that received only ribavirin, that the addition of lopinavir-ritonavir (400 mg and 100 mg ...
Placebos without Deception: A Randomized Controlled Trial in ... - PLOS Background Placebo treatment can significantly influence subjective symptoms. However, it is widely believed that response to placebo requires concealment or deception. We tested whether open-label placebo (non-deceptive and non-concealed administration) is superior to a no-treatment control with matched patient-provider interactions in the treatment of irritable bowel syndrome (IBS). Methods ...
CANNABIDIOL (CBD) - Uses, Side Effects, and More - WebMD CBD can cause some side effects, such as dry mouth, low blood pressure, light headedness, and drowsiness. Signs of liver injury have also been reported with high doses of the prescription form of ...
Open-label extension studies: do they provide meaningful ... If undertaken primarily to gather more patient-years of exposure to the new drug in order to understand and gain confidence in its safety profile, open-label extension studies can play a useful and legitimate role in drug development and therapeutics. However, this can only occur if the open-label extension study is designed, executed, analysed ...
Audience Is Everything™ Audiencemeasurement. Know everything about your audiences with our cross-platform measurement data of the entire population and its shifting habits. Explore.
The Lancet Oncology In this study, the 5-year locoregional recurrence rate was less than 4%, which supports our hypothesis that it is oncologically safe to de-escalate locoregional radiotherapy based on locoregional recurrence risk, in selected patients with cT1-2N1 breast cancer treated with primary chemotherapy, according to this predefined, consensus-based ...
Hydroxychloroquine and azithromycin as a treatment of COVID-19: results ... Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. ... Twenty cases were treated in this study and showed a significant reduction of the viral carriage at D6-post inclusion compared to controls, and much lower average carrying duration than reported in the litterature for ...
JAMA Network | Home of JAMA and the Specialty Journals of the American ... JAMA Network Open. Explore the latest research in hypertension, diabetes, stroke, dementia, machine learning, and more all completely free and open access. ... This Global Burden of Disease study estimates changes in prevalence of major neurological disorders in the US by age and sex between 1990 and 2017.
Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers and the ...
H-E-B | Curbside Pickup & Grocery Delivery | HEB.com H-E-Buddy Sugar Free Kids Multi Vitamin Jelly Beans. Add to cart. Add to list. $5.03 each ($0.08 / ct) H-E-B Calcium with Vitamin D Gummy Kid Gummies Vitamins. Add to cart. Add to list. $8.98 each ($0.30 / ct) H-E-B Vitamins 1000 mg Vitamin C Orange Powder Drink Mix. Add to cart. Add to list.
Effect of Dexamethasone in Hospitalized Patients with COVID-19 ... Methods The Randomised Evaluation of COVID-19 therapy (RECOVERY) trial is a randomized, controlled, open-label, adaptive, platform trial comparing a range of possible treatments with usual care in patients hospitalized with COVID-19. We report the preliminary results for the comparison of dexamethasone 6 mg given once daily for up to ten days vs. usual care alone.
























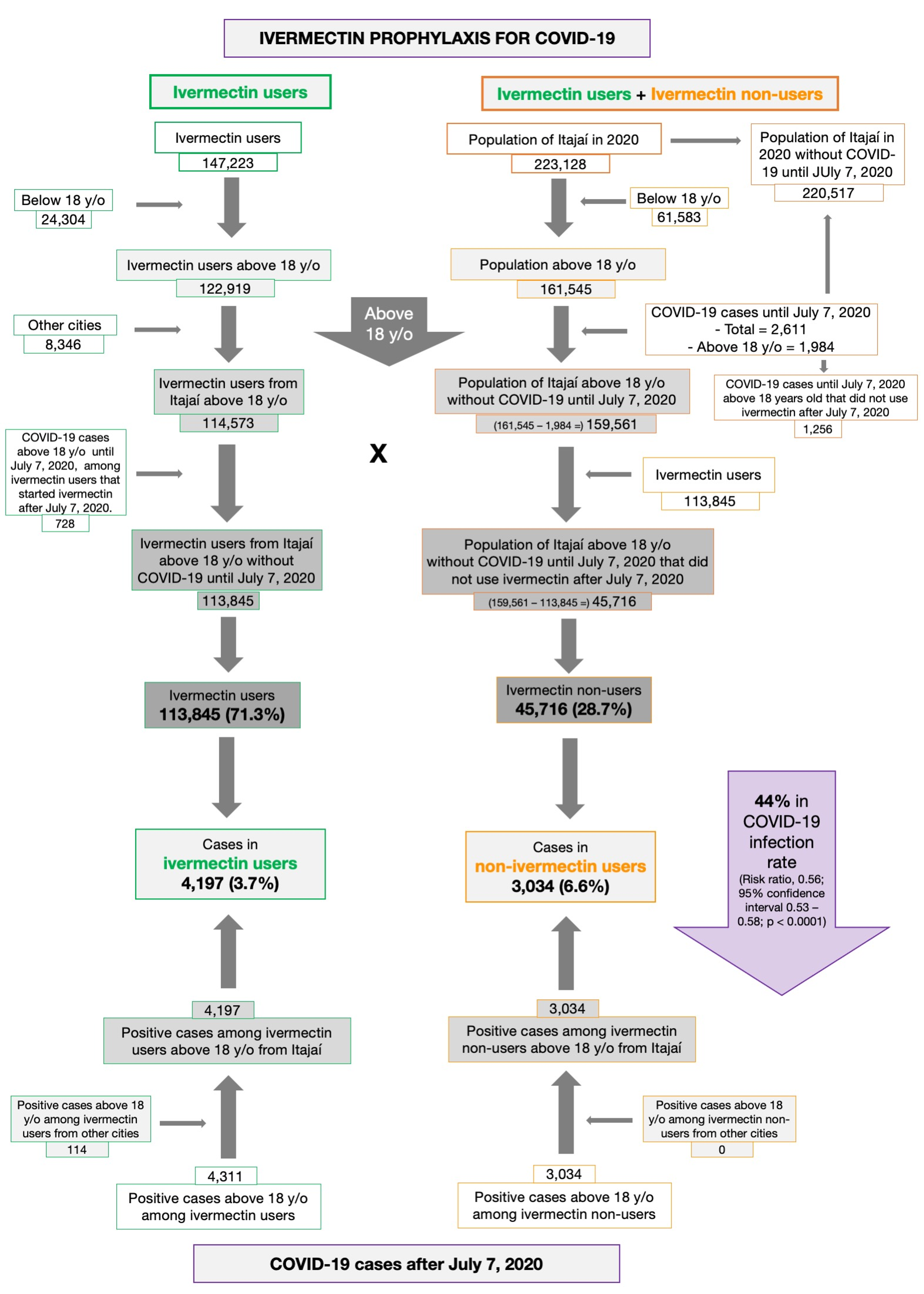



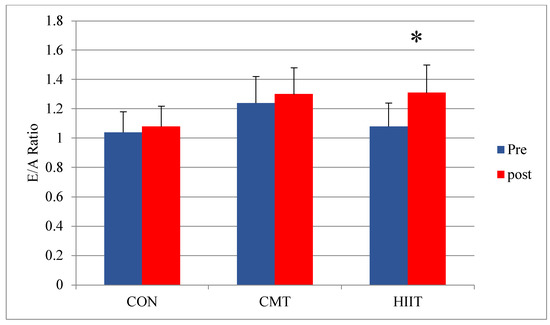


Post a Comment for "40 open-label study o que é"